Vaccine candidates developed by Turkish scientists are closer to reality as some enter the last phase of trials and others proceed with success in the early stages. The country hopes to have its own vaccine available by the end of this year or earlier.
The closest to finalization is a vaccine developed by Erciyes University in the central province of Kayseri and it will switch to Phase 3 trials in May. The inactive vaccine is among 18 developed by the country’s scientific community. Turkey’s vaccine studies are endorsed by the Scientific and Technological Research Council of Turkey (TÜBITAK) and the Presidency of Turkish Health Institutes (TÜSEB).
With locally made jabs, authorities hope to eliminate the dependence on vaccine imports and provide a reliable alternative to the public, as foreign-made vaccines occasionally face skepticism. Currently, CoronaVac from China and Comirnaty developed by Pfizer and BioNTech are available in Turkey. Health Minister Fahrettin Koca announced on Thursday that they would start administering Russia’s Sputnik V vaccine soon.
Erciyes University’s vaccine started Phase 2 trials in February, about two months after Phase 1 trials. A total of 294 volunteers joined the two trials.
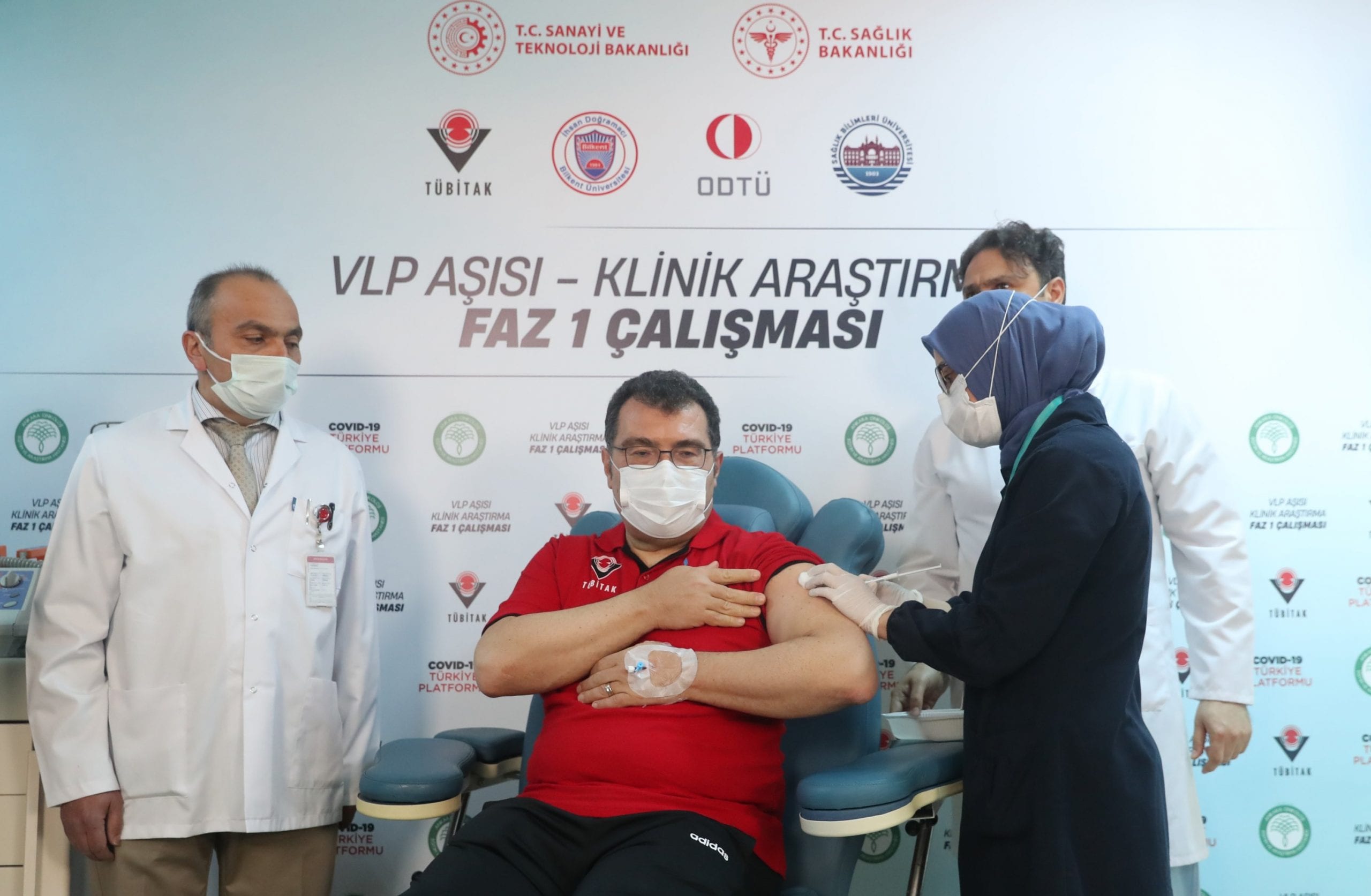
In the meantime, clinical trials involving the administering of the first doses of a virus-like particle (VLP) based locally produced COVID-19 vaccine on volunteers have been completed. Second doses are expected to be administered in May. Fevzi Altuntaş, the chief physician at the Ankara Oncology Training and Research Hospital in the Turkish capital, is the coordinator of the center where the vaccine is tested. “We finished the first doses. We started administering the second doses. It is also proceeding quickly. If there are no setbacks, we will have completed these doses in the first week of May,” he told Anadolu Agency (AA) on Thursday.
Altuntaş said they received official approval from the Health Ministry’s Medicines and Medical Devices Institution as of March 19, adding the first volunteers had been included in the clinical study starting from March 26. He said people over the age of 18 who do not have any health problems and have not had COVID-19 before can volunteer for the vaccine study.
Altuntaş said they administered the vaccine in two doses and monitored its safety for six months, stressing that the first 24 hours in this process are very valuable, so the volunteers are closely monitored both by physical examination and in the laboratory. He said one of the volunteers in the vaccine trials was the Industry and Technology Minister Mustafa Varank.
Mentioning that both Varank and Hasan Mandal, president of TÜBITAK, closely monitored the studies, Altuntaş said “they were both suitable for the clinical studies as they tested negative. So they stayed here for 24 hours and witnessed nothing serious after the process.” He added, “Varank told us he experienced some aching in his arm, but Mandal did not tell us anything.”
He said that the vaccine was an innovative product, noted that when a mutation develops, the vaccine can be designed according to it and adapted quickly.
The Phase 2 trial will start with more than 200 volunteers and scientists plan to start it in the first week of June. Asked when the vaccine could be released, Altuntaş said, “If all goes well, it could be ready for citizens’ use in the autumn.”